The Babraham Institute has devised a mechanism to “time hop” human skin cells by 30 years, effectively turning back the clock for cells without affecting their specialized function. Researchers from the institute’s epigenetics research department have been able to partially repair the function of aged cells while also regenerating molecular measurements of biological age. The study was published today in the journal eLife, and while it is still in its early stages of development, it has the potential to revolutionize regenerative medicine.
What exactly is regenerative medicine?
Our cells’ ability to function reduces as we age, and the genome collects signs of aging. Regenerative biology seeks to repair or replace cells, including those that have died. Our ability to generate “induced” stem cells is one of the most essential tools in regenerative biology. The procedure consists of numerous phases, each of which removes some of the markers that differentiate cells. In theory, these stem cells have the capacity to become any cell type, but scientists have yet to successfully reproduce the conditions that allow stem cells to re-differentiate into all cell kinds.
Rewinding time
The new procedure, which is based on Nobel Prize-winning technology used by scientists to create stem cells, addresses the challenge of completely erasing cell identity by stopping reprogramming part way through the process. This enabled researchers to strike the perfect balance between reprogramming cells, making them biologically younger, and restoring their specialized cell functions.
Age is more than just a number.
The researchers sought alterations in the signs of aging to demonstrate that the cells had been revived. As Dr Diljeet Gill, a postdoc in Wolf Reik’s lab at the Institute who conducted the research as a PhD student, explained: “Over the last decade, our understanding of ageing at the molecular level has advanced, giving rise to techniques that allow researchers to measure age-related biological changes in human cells. We were able to apply this to our experiment to determine the degree of reprogramming achieved by our novel approach.
“Our understanding of ageing on a molecular level has progressed over the last decade, giving rise to techniques that allow researchers to measure age-related biological changes in human cells. We were able to apply this to our experiment to determine the extent of reprogramming our new method achieved.”
Dr Diljeet Gill,
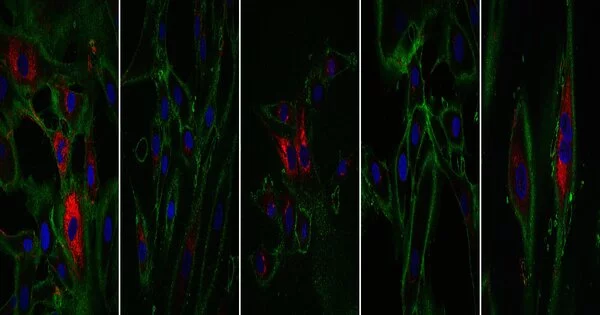
The researchers looked at a variety of cellular age measurements. The first is the epigenetic clock, which uses chemical markers found throughout the genome to determine age. The second component is the transcriptome, which contains all of the gene readouts produced by the cell. When compared to reference data sets, the reprogrammed cells matched the profile of cells that were 30 years younger by these two metrics.
The prospective applications of this technology rely on the cells’ not just appearing younger, but also functioning as young cells. Fibroblasts produce collagen, a substance present in bones, skin, tendons, and ligaments that aids in tissue structure and wound healing. When compared to control cells that did not go through the reprogramming process, the rejuvenated fibroblasts produced more collagen proteins. Fibroblasts also migrate to places that require healing. The partially regenerated cells were examined by making an artificial cut in a layer of cells in a dish. They discovered that treated fibroblasts moved faster into the gap than older cells. This is a positive hint that one day this study could be utilized to make cells that repair wounds more effectively.
This research could lead to new therapy options in the future; the researchers discovered that their technique had an influence on additional genes connected to age-related disorders and symptoms. Both the APBA2 gene, which has been linked to Alzheimer’s disease, and the MAF gene, which has been linked to the formation of cataracts, showed alterations toward younger levels of transcription.
The mechanism underlying successful transitory reprogramming is yet unknown, and this is the next piece of the jigsaw to be solved. The researchers think that crucial portions of the genome involved in cell identity formation may be spared from the reprogramming process.
Diljeet ended by saying, “Our findings reflect a significant advancement in our understanding of cell reprogramming. We have demonstrated that cells can be renewed without losing function and that rejuvenation attempts to restore some function to elderly cells. The fact that we also detected a reversal of ageing signs in genes related to illnesses is really encouraging for the future of this research. “
Professor Wolf Reik, who just took over as director of the Altos Labs Cambridge Institute after serving as a group leader in the epigenetics research program, stated: “The ramifications of this finding are really interesting. We may eventually be able to discover genes that regenerate without requiring reprogramming, and particularly target those to minimize the consequences of aging. This technique has the potential to yield crucial discoveries that could pave the way for a fantastic therapeutic horizon. “





