A method that may help identify Parkinson’s disease symptoms in urine samples has been developed by a group led by researchers at Purdue University and Tymora Analytical Operations, a spinoff of Purdue.
LRRK2 (leucine-rich repeat kinase 2) proteins, which are linked to Parkinson’s disease, and their downstream pathways may be altered in Parkinson’s patient samples using this method. The strategy could ultimately prompt far and wide painless testing for other neurodegenerative circumstances as well as disease.
W. Andy Tao, a professor of biochemistry at Purdue, stated, “We believe this is a logical and rational approach to move forward for the diagnosis of Parkinson’s disease.” Conclusion for this kind of neurodegenerative illness is troublesome.” Mental and development testing can require a year or more to affirm the determination, so sub-atomic tests for early conclusion and mediation can assist individuals with Parkinson’s quicker, he made sense of.
In the Communications Medicine journal, Tao and eight co-authors from Purdue, Tymora, The Michael J. Fox Foundation for Parkinson’s Research, and Columbia University presented their findings.
“Especially for neurodegenerative diseases and cancer,” Tymora’s president and chief technology officer and co-author Anton Iliuk predicted, “it’s going to be a big new area in diagnostic development.”
Only Parkinson’s disease is thought to affect 1 percent of people over 60. The disease affects up to one million Americans, and 90,000 new cases are diagnosed each year.
The paper’s co-creators incorporate Marco Hadisurya, a doctoral understudy in natural chemistry; a doctoral student in electrical and computer engineering named Kananart Kuwaranancharoen; Xiaofeng Wu, who accepted his doctorate in science at Purdue in 2022; Li, Tymora Scientific Activities; West Lafayette Junior/Senior High School’s Zheng-Chi Lee; Roy Alcalay, MD, Columbia College; and The Michael J. Fox Foundation’s Shalini Padmanabhan.
Padmanabhan suggested working together after reading some of Iliuk and Tao’s research on the EVtrap (Extracellular Vesicles total recovery and purification) method for urinary analysis.
Padmanabhan stated, “It was interesting to note the expression of an important Parkinson’s disease-linked protein, LRRK2 when I reviewed the data from their previous publication.” This provoked my curiosity since this approach gave us a valuable chance to decide whether LRRK2 proteins or the downstream pathways they influence are really changed in urinary examples from Parkinson’s patients who harbor a transformation in the quality.”
In 2017, Tao drove a group that fostered a blood test that could possibly distinguish bosom disease. Tao and his colleagues compared breast cancer patient and healthy control group samples in that work.
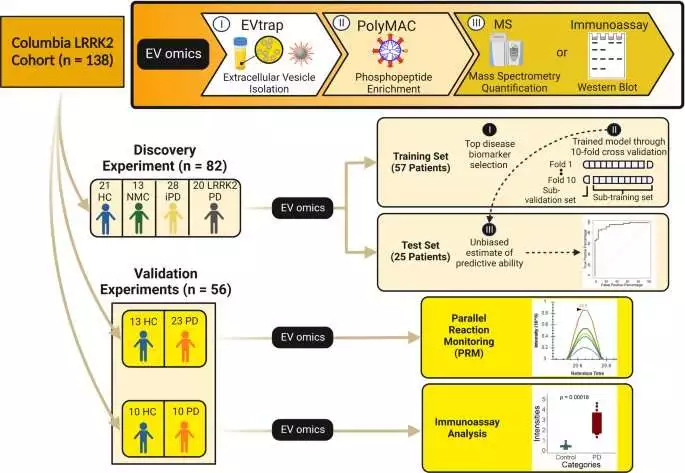
the creation and testing of biomarker signatures for Parkinson’s disease diagnosis. Credit: Communications and Medicine DOI: 10.1038/s43856-023-00294-w
“We recognized phosphorylated proteins, which are a common sign of malignant growths,” Tao said. The researchers also discovered extracellular vesicles, which are small packages that cells use as their molecular delivery system, within these proteins. The result showed that phosphoproteins in a blood sample could be used as a possible indicator for early cancer diagnosis or disease progression.
Using Tymora’s EVtrap method, the team was able to quickly separate the vesicles from urine samples.
According to Iliuk, who earned his doctorate in biochemistry from Purdue in 2011, “we have used the method for a number of indications, primarily focusing on different cancers for biomarker discovery and validation.” Tymora Analytical, which specializes in biofluid biomarker detection technology and services, was co-founded by Iliuk and Tao.
“This sort of examination opens another outskirts in painless diagnostics advancement. It’s showing that biomarkers recently remembered to be imperceptible have become uncovered and do a truly great job of separating illness from non-infection state,” Iliuk said. ” Clearly pee would be a wellspring of mind based synthetic compounds or marks, yet it is. The blood-brain barrier can be easily broken by these EVs.
After send out from the mind into the circulatory system, they become moved or separated into pee. In any case, testing such biomarkers from the mind through spinal tap is a profoundly obtrusive method.
“Particularly for early conclusion that isn’t the favored examining technique,” Tao said. While some proteins in urine samples carry out non-disease-related housekeeping tasks, others could serve as disease markers.
“Extracellular vesicles give a method for zeroing in on sickness markers since they are delivered by specific sorts of cells,” he said.
Among the numerous ways of concentrating on the effect of LRRK2 is to follow its organic pathway, which should be possible by dissecting pee, blood and cerebrospinal liquid. An easy way to monitor changes in urine, which is collected for many clinical studies, was provided by the EVtrap method.
For useful Parkinson’s disease research, Columbia University’s LRRK2 Biobanking Study has a large bank of urine samples. Alcalay, a co-author from Columbia University who provided many of the samples, also contributed to the correlation of the EVtrap data with the clinical data. The team looked at samples from patients with and without the disease and from people with and without the LRRK2 gene mutation for the Communication Medicine study.
“This study also highlighted that changes in urinary proteins could serve as a proxy for changes in protein signatures that occur in brain diseases such as Parkinson’s disease,” Padmanabhan stated.
A connection between LRRK2 and brain proteins in urine samples was demonstrated in a 2021 paper by an international team of researchers that was published in the journal EMBO Molecular Medicine. Padmanabhan and Alcalay of Columbia University served as co-authors on that study, which was directed by Matthias Mann of the Max Planck Institute of Biochemistry in Germany.
More information: Marco Hadisurya et al, Quantitative proteomics and phosphoproteomics of urinary extracellular vesicles define putative diagnostic biosignatures for Parkinson’s disease, Communications Medicine (2023). DOI: 10.1038/s43856-023-00294-w





