In a study that was published in Nature Communications, a group led by Senior Scientists at the Krembil Brain Institute at UHN, Drs. A protein-protein interaction that contributes to Parkinson’s disease has been identified by Lorraine and Suneil Kalia, as well as Dr. Philip M. Kim, a professor at the University of Toronto (U of T).
In the sickness, a protein called α-synuclein (a-syn) gathers in the mind and prompts cell demise. Currently, a lot of research is focused on using antibodies to clear a-syn or small molecules to stop a-syn from aggregating. In this study, the researchers took a different approach by looking for protein-protein interactions that might help Parkinson’s disease patients accumulate a-syn.
The cell’s inner workings, including the breakdown of disease-causing proteins, are governed by interactions between proteins. A promising strategy for treating diseases like cancer and stroke is inhibiting specific interactions.
“Identifying a specific interaction that contributes to a disease and then finding ways to disrupt it can be a time-consuming and arduous process,”
Dr. Lorraine Kalia, who is also a staff neurologist at UHN .
Dr. Lorraine Kalia, who is also a staff neurologist at UHN and a scientist at the Tanz Centre for Research in Neurodegenerative Diseases in the Temerty Faculty of Medicine at the University of Toronto, says, “Identifying a particular interaction that contributes to a disease and then finding ways to disrupt it, can be a painstaking and incredibly slow process.”
According to Dr. Kalia, “We all started out a little bit skeptical that we would have something useful at the end, and so the fact that we do have something that warrants further work is much more than we anticipated”
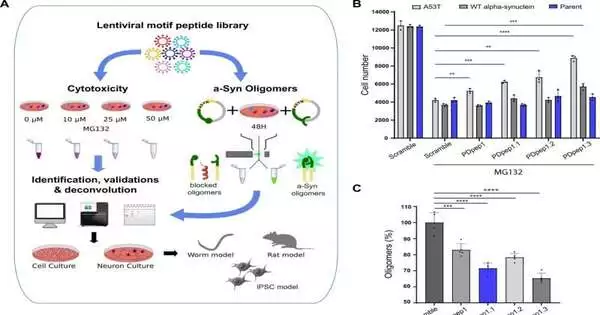
Dr. Kim claims that the team took the opposite approach in order to speed up the discovery of potential therapies: We fostered a stage to screen particles called peptide themes — short strings of amino acids that can disturb protein connections — for their capacity to safeguard cells from a-syn. Following the identification of potential peptides, the protein-protein interactions they target were identified.
Through this methodology, the group recognized a peptide that decreased a-syn levels in cells by upsetting the cooperation between a-syn and a protein subunit of the phone hardware called endosomal arranging complex expected for transport III (ESCRT-III).
“ESCRT-III is a part of a pathway that phones use to separate proteins, called the endolysosomal pathway,” makes sense of Dr. Lorraine Kalia. ” We found that a-syn communicates with a protein inside ESCRT-III — CHMP2B — to hinder this pathway, consequently forestalling its own obliteration.
“We were dazzled that the stage worked,” she adds. ” However, the fact that we were able to discover an interaction that had not really been characterized previously and a pathway that had not yet been targeted for therapeutics through this type of screening is what I believe was more intriguing.
Dr. Suneil Kalia says that once the team found this interaction, they proved they could use their peptide to break it, stopping a-syn from getting past the cell’s natural clearance pathways.
“We tried the peptide in various exploratory models of Parkinson’s illness, and we reliably found that it reestablished endolysosomal capability, advanced a-syn freedom and forestalled cell passing,” he said.
These discoveries demonstrate that the a-syn-CHMP2B cooperation is an expected remedial objective for the sickness, as well as different circumstances that include a development of a-syn, for example, dementia with Lewy bodies.
The following stages for this examination are to explain precisely how a-syn and CHMP2B connect to disturb endolysosomal action. Progressing studies are additionally deciding the best methodology for conveying possible therapeutics to the cerebrum.
“This exploration is still in its beginning phases — more work is certainly expected to make an interpretation of this peptide into a reasonable restorative,” alerts Dr. Lorraine Kalia. ” Despite this, our findings are very exciting because they point to a new avenue for the development of Parkinson’s disease and other neurodegenerative conditions treatments.
The significance of multidisciplinary collaborations in health research is also emphasized in this study.
Dr. Suneil Kalia asserts, “We simply could not have conducted this study in a silo.” Due to the lack of research on the endolysosomal pathway, it was difficult to determine where to look for potential disease-related protein-protein interactions. The screening platform provided by Dr. Kim was crucial in directing us in the right direction.”
“It is exceptional to see this stage — which we at first used to track down expected therapeutics for disease — yielding advances in mind research. “The insights that we gain about one organ system or disease could have important implications in other contexts because the pathways that cells use to stay healthy are fundamentally very similar across tissues,” says Dr. Kim.
According to Dr. Lorraine Kalia, “This is our first collaboration with Dr. Kim, and it has been a productive one with a lot of synergy.” We hope this will accelerate Parkinson’s research by looking for technologies that are utilized in other fields and applying them to our own.
She goes on to say, “It’s really brand new science and brand new targets that haven’t been a focus for Parkinson’s drug development.” We hope this changes the treatment landscape for this disease, which so desperately requires new therapies.”
More information: Satra Nim et al, Disrupting the α-synuclein-ESCRT interaction with a peptide inhibitor mitigates neurodegeneration in preclinical models of Parkinson’s disease, Nature Communications (2023). DOI: 10.1038/s41467-023-37464-2