UT Southwestern scientists report in another review that a bunch of connecting particles in safe cells of the stomach are liable for forestalling the irritation seen in fiery gut illnesses (IBD). The discoveries, published in Cell Reports, propose another medication focus for treating IBD and related conditions.
“We found a key system that hinders irritation in the stomach,” said Venuprasad Poojary, Ph.D., academic partner of Inner Medication and Immunology at UT Southwestern and an individual from the Harold C. Simmons Complete Disease Place. “Understanding these sorts of fundamental insights regarding the safety framework is fundamental for developing new systems to treat fiery illnesses.”
“We clearly shown that activating this pathway can decrease colon inflammation, and we believe this pathway could be targeted to reduce inflammation as well.”
Dr. Poojary
Fiery gut illnesses, which incorporate Crohn’s sickness and ulcerative colitis, are described by ongoing and extreme irritation of the gastrointestinal tract. Medicines for these illnesses exist, yet frequently stifle the safe framework all through the body, prompting aftereffects and an expanded risk of disease. Drugs that more explicitly focus on the stomach are a longstanding objective.
Analysts definitely realize that uplifted levels of the safe atom IL-17 are related to the most awful side effects of IBD. Yet, while drugs focusing on IL-17 have been created for psoriasis, they have been incapable of treating IBD; they likewise represent the issue of affecting safe cells all over the body.
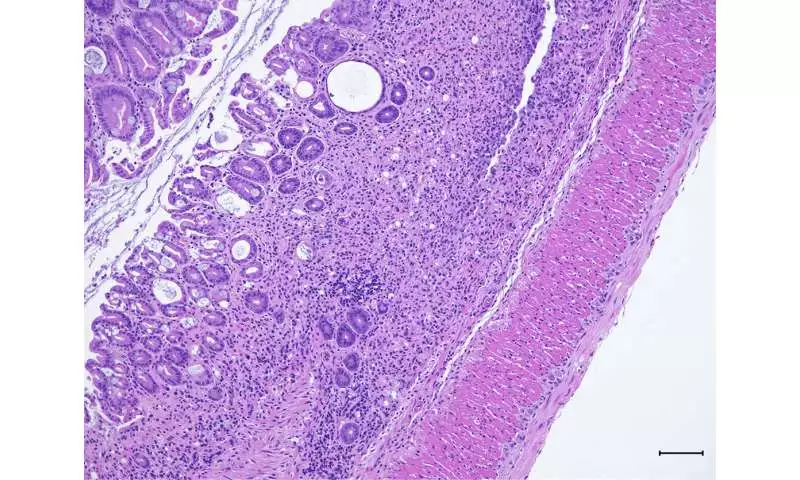
Histology reveals colon tissue damage in mice lacking Pak2.
In the new review, Dr. Poojary and his partners tested what different atoms connect in the fiery cells that produce IL-17 in the stomach. Their tests turned up a protein called Pak2. When the analysts impeded Pak2 in mice, the creatures shed pounds, had more colon irritation, and showed different side effects of IBD, remembering the runs and blood in their stools. Within the sight of Pak2, nonetheless, the IBD-like irritation was facilitated.
Further tests uncovered that Pak2 is tied to RORgt, a protein that enacts the IL-17 quality. Yet, while RORgt behaves like a gas pedal for irritation by helping levels of IL-17, Pak2 behaves like the brakes. Dr. Poojary’s group showed that Pak2 acts to debase RORgt and keep it from enacting the IL-17 quality.
“We plainly demonstrated the way that turning up this pathway can hinder colon irritation, and we figure this pathway could be designated to lessen this aggravation too,” said Dr. Poojary.
His lab is currently arranging follow-up examinations to foster medications that could support the Pak2 brakes on irritation. The examination could have suggested past IBD, he added.
“Despite the fact that we concentrated on this with regards to gut illnesses, we think this pathway is likely likewise relevant to other fiery infections, including various sclerosis and rheumatoid joint pain,” he said.
More information: Mahesh Kathania et al, Pak2-mediated phosphorylation promotes RORγt ubiquitination and inhibits colonic inflammation, Cell Reports (2022). DOI: 10.1016/j.celrep.2022.111345
Journal information: Cell Reports