The molecular processes that first take place in the eye when light hits the retina have been deciphered by researchers at the Paul Scherrer Institute (PSI). The procedures, which only take a trillionth of a second, are necessary for human vision. The study was recently published in Nature, a scientific journal.
It only involves a minute modification of a protein in our retina, and this modification takes place in a very brief amount of time. It is the very first step in our ability to perceive light. It is additionally the main light-subordinate step. Using the PSI’s SwissFEL X-ray free-electron laser, PSI researchers have determined precisely what happens after the first trillionth of a second in the process of visual perception.
Our light receptor, the protein rhodopsin, is at the center of the action. It is produced by rod cells, sensory cells that are specialized in detecting light, in the human eye. A small, kinked molecule is held in place in the middle of the rhodopsin: retinal, a subsidiary of vitamin A. At the point when light raises a ruckus around town, the retina retains part of the energy. It then transforms into a three-dimensional form at breakneck speed, flipping the eye switch from “off” to “on.” A series of reactions are sparked by this, culminating in the perception of a flash of light.
“The SwissFEL enables us to examine the fundamental processes of the human body in great detail, such as vision,”
Gebhard Schertler, Head of PSI’s Biology and Chemistry Research Division.
Tied but free, but exactly what happens when the retina changes from the 11-cis form to the all-trans form? According to PSI scientist Valérie Panneels, “We have known about the starting point and the end product of the retinal transformation for some time, but so far no one has been able to observe in real time exactly how the change occurs in the sight pigment rhodopsin.”
Panneels likens the process to a cat falling backwards from a tree and somehow surviving to stand on its own. The inquiry is: What position does the cat assume when it falls before rising to its feet?
The PSI researchers discovered that the “retinal cat” turns its middle part first. The “eureka moment” for Valerie Panneels came when she realized something else that happens: “like our chest expanding when we breathe in, only to contract again shortly afterwards,” the protein does when it takes in some of the light energy.
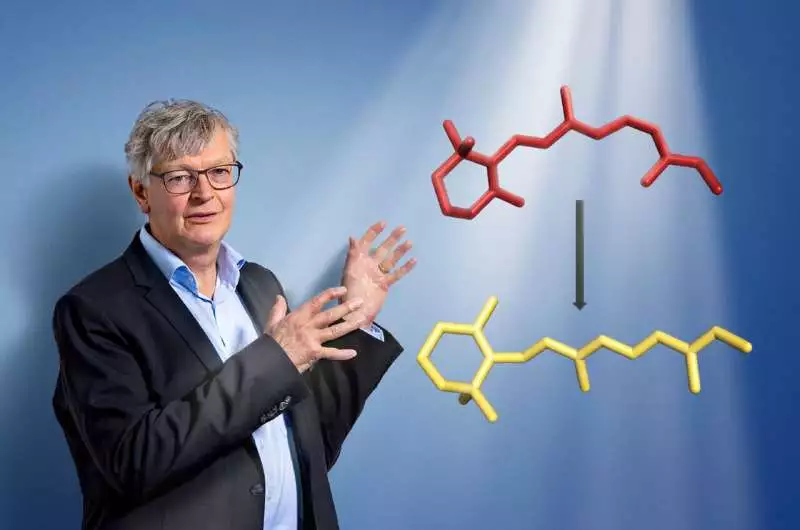
The head of PSI’s Biology and Chemistry Research Division is Gebhard Schertler. The retinal molecule, which changes shape when light enters our eyes and initiates the visual process, is also depicted in the image.
The protein temporarily loses most of its contact with the retina in its middle during this “breathing in” stage. The retinal can now rotate, even though chemical bonds keep it connected to the protein at its ends. The molecule resembles a dog on a loose leash who is free to jerk at any time at that moment.
The protein contracts once more shortly after and regains control of the retina, this time in a different, more elongated form. The retina’s ability to turn itself is not hindered by the protein that holds it in place.
One of the fastest natural processes The retina’s transformation from its 11-cis kinked form into its all-trans elongated form takes only one picosecond, or one trillionth of a second (10–12). This makes it one of the fastest natural processes.
An X-ray free-electron laser like the SwissFEL is the only method for recording and analyzing such rapid biological processes. According to Gebhard Schertler, Head of PSI’s Biology and Chemistry Research Division and joint lead author of the study with Valérie Panneels, “The SwissFEL enables us to study in detail the fundamental processes of the human body, such as vision.”
To return to the cat’s analogy, this would be similar to using a high-speed camera to record its fall, with one major difference: The SwissFEL camera has a million times faster filming speed. There is a lot more to working with large research facilities than just pressing a shutter button. Thomas Gruhl, a Ph.D. student who went on to work as a postdoctoral researcher at the Institute for Structural and Molecular Biology in London, worked for years to develop a method for producing high-quality rhodopsin crystals that can provide data with ultra-high resolution. In the end, only these data made it possible to make the necessary measurements at SwissFEL and at the X-ray free-electron laser SACLA in Japan before SwissFEL was built.
Once more, this examination shows SwissFEL’s crucial job in Swiss exploration. ” According to Gebhard Schertler, “it will probably help us come up with many more solutions in the future.” We are also working on ways to study dynamic processes in proteins that are normally not activated by light, among other things.” The researchers utilize counterfeit means to make such atoms receptive to light: possibly they roll out proper improvements to the limiting accomplices, or they blend proteins in with restricting accomplices in the gem so rapidly that they can be analyzed at the SwissFEL. In any case, merely pointing a camera at a cat falling from a tree doesn’t cut it when it comes to the procedure at hand.
More information: Valerie Panneels, Ultrafast structural changes direct the first molecular events of vision, Nature (2023). DOI: 10.1038/s41586-023-05863-6. www.nature.com/articles/s41586-023-05863-6





