Examining how proteins are altered after they have been translated can give us a better understanding of cellular processes. A brand-new single-molecule detection assay that can accomplish this utilizing electrical measurements has been created by researchers at Tokyo Tech. The method had a 91% specificity and 95% accuracy in detecting the phosphorylation of peptides.
Our body’s cells engage in millions of biological operations daily. Investigating these processes can teach us more about how cells work, a topic that has long piqued academics’ interest.
Recently, however, there has been a new player in this field. Single-molecule detection is a new analytical technique that has gained popularity because it is effective in identifying particular, physiologically significant molecules and the processes connected to them.
Researchers have explored methods for analyzing proteins and their post-translational modifications (PTMs) using single-molecule detection techniques. After protein synthesis, functional groups are added to the amino acids in the protein to give it a specific function. This process is known as PTM.
The study of PTMs can help us understand cell signaling and the origin of several diseases. However, assays aiming to do so have to be highly selective and specific to that protein. It is difficult to acquire single-molecule PTM measurements due to the low sensitivity of current methods.
There is a strong connection between protein phosphorylation and the pathogenesis of a wide range of diseases. Our method will allow scientists to detangle how phosphorylation regulates the cellular events that lead to the origin of a disease and thereby aid in the development of treatments.
Professor Tomoaki Nishino
Tokyo Institute of Technology (Tokyo Tech) researchers have discovered a “electrifying” solution to these constraints. In their recent breakthrough, published in the Journal of the American Chemical Society, a team of scientists led by Associate Professor Tomoaki Nishino from Tokyo Tech reported the single-molecule detection of phosphorylation in peptides short amino acid chains and the formation of an orthophosphate junction with the help of electronic signatures.
Dr. Nishino explains, “We chose peptide phosphorylation, an archetypal and biologically relevant PTM, for our detection studies. The aim was to develop a tool that could detect even the slightest alteration in the chemical structure of amino acids.”
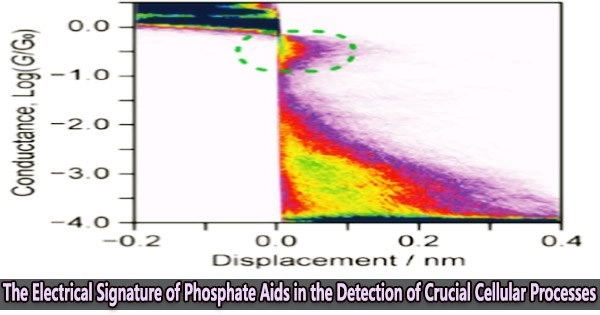
To start with, the team studied the electronic properties of phosphorylated peptides using their inorganic analog, orthophosphoric acid (H3PO4). They prepared a phosphate solution (PO43-) and subjected it to a scanning tunneling microscope (STM)-assisted break-junction (BJ) technique.
An orthophosphate group was discovered to bridge the nanogap between the electrodes by producing a stable junction as a result of its negatively charged oxygen atoms interacting with the gold when the current was transferred between two gold STM electrodes. It was this junction and its signature that drove further experiments.
The high conductance of 0.4 G0 and distinctive electrical characteristics of the single-orthophosphate junction were discovered, and it was these characteristics that made it possible for this process to be extremely precise and accurately sense the PTM in question (i.e., phosphorylation).
The team used in situ single-molecule phosphorylation assays to further validate their method and found that they could distinguish between phosphorylated and non-phosphorylated peptides with 95% accuracy and 91% specificity.
The approach used in this study offers an unexpected window into the world of PTMs in proteins. Also, this innovative method will provide new opportunities for the application of single-molecule PTM detection in clinical diagnosis and pharmaceutical applications.
“There is a strong connection between protein phosphorylation and the pathogenesis of a wide range of diseases. Our method will allow scientists to detangle how phosphorylation regulates the cellular events that lead to the origin of a disease and thereby aid in the development of treatments,” concludes Dr. Nishino.