Spiculogenesis, or the formation of glass-like structures, and biomineralization in early sponge species have resulted in the natural occurrence of very regular glass architectures. In a new article published in Advanced Science, Hermann Ehrlich and a group of worldwide experts studied a previously unknown role of the protein actin.
To answer a fundamental question, the team of biomineralogists used current bioanalytical technologies to demonstrate the presence of the protein actin in the glass structures of sponges. In both prokaryotes and eukaryotes, actin is a crucial member of an ancient superfamily of structural intracellular proteins that play an important role in the cell’s cytoskeleton and cellular dynamics. In metazoans, the protein has other activities, such as patterning biosilica deposition lasting more than 500 million years. The scientists demonstrated how axial filaments are patterned in the silica architecture of metazoans in vivo, answering a basic question in bioinspired biomaterials science on the growth of “biological glass.” The researchers also demonstrated why metazoans evolved to meter-sized sizes at temperatures ranging from -1.9 °C to 24 °C.
“one of the most thoroughly studied fundamental proteins that still holds surprises. … recent findings showed how actin played a new role to organize the pattern of the skeleton to the most basal of animals—the sponges.”
Hermann Ehrlich, professor and head of the Biomineralogy and Extreme Biomimetics research group at the Freiberg University of Mining and Technology in Germany,
Actin is full of unexpected twists and turns.
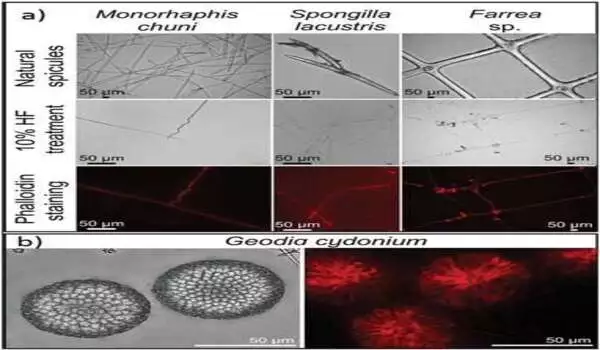
Hermann Ehrlich, the study’s lead author and head of the Biomineralogy and Extreme Biomimetics research group at the Freiberg University of Mining and Technology in Germany, described the process as “one of the most thoroughly studied fundamental proteins that still holds surprises.” Recent findings show how actin plays a new role in organizing the pattern of the skeleton in the most primitive of animals—the sponges. The researchers defined their technique as “an endeavor to observe what everyone has seen and to think what nobody has thought.” Ehrlich et al. used a variety of bioanalytical techniques to complete their ambitious research plan on the role of actin in marine sponges, including proteomics, Western blotting, phalloidin staining, immunostaining, high-resolution transmission electron microscopy, Raman spectroscopy, and Fast Fourier transform techniques. Using these methods, the researchers demonstrated that F-actin was used to make axial filaments in a variety of typical sponge classes, including Hexactinellida and Demospongiae. “Amazingly, even the gigantic axial filaments of the giant glass sponge Monorhaphis chuni are made up of F-actin, revealing the largest actin filament bundles ever observed,” Ehrlich added.
A traditional role with a contemporary twist.
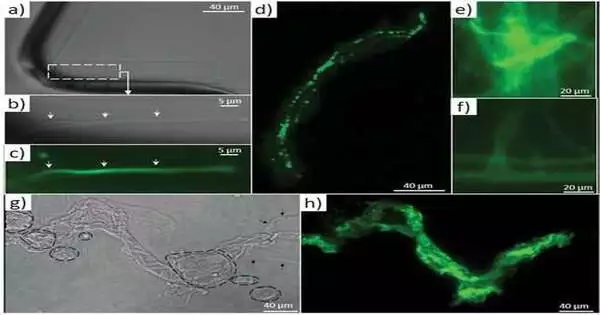
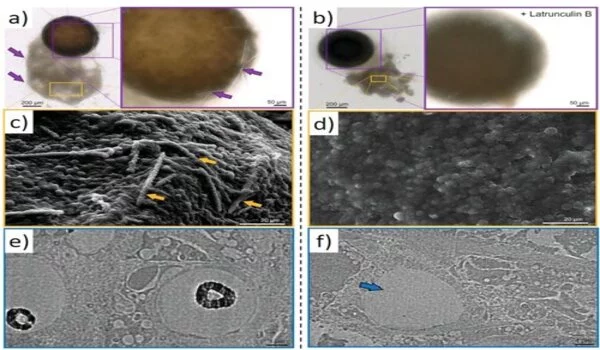
The researchers emphasized how sponge biomaterials’ maintained shape describes an old role that F-actin has had for at least 545 million years. In their investigation, Ehrlich et al. hypothesized that F-actin could be found in an ancestral intracellular siliceous construct space. The materials may have been transferred to extracellular spaces via the formation of glass-like structural particles known as spicules or spiculogenesis, where actin continued to perform a pattern-forming role. According to the researchers, actin within such glass formations appears to serve a new function as a driving force in defining the diversity of advanced biosilica structures in sponges. During the investigation, the researchers devised a new microscopic method for isolating axial filaments from siliceous microstructures produced by a variety of sponge species. A drop of 10% hydrofluoric acid was carefully placed on the surface of a spicule to allow the acid to dissolve the biosilica while leaving the organic matrix intact. Ehrlich et al. found actin in the axial filaments of a number of sponges using this method and immunofluorescence labeling.
The actin filaments are being studied.
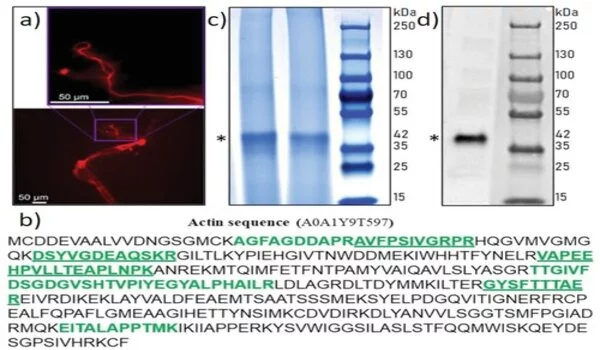
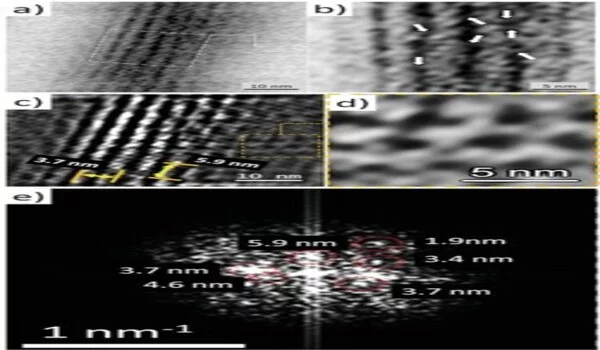
The researchers employed a variety of tests, including proteomics, to demonstrate actin as a component of axial filaments, as well as the presence of actin in glass sponge spicules. They also used high-resolution transmission electron microscopy to see and understand the periodic structure of actin, followed by Fourier transform imaging to figure out the structure of these crystals, all while looking into the nanostructural organization of axial filaments, which is typical of F-actin. They next used inhibition experiments to block spicule formation in order to investigate the involvement of actin during spicule development in vivo. The findings backed up a recent study that found that disrupting the actin network interfered with the release of biomineralized components in marine unicellular haptophytes. The research findings, according to Ehrlich et al., “will have an impact on research in a varied variety of disciplines, including bioinspired materials science, biomechanics, biomineralization, the chemistry of science, functional materials, and biomimetics.”
The future of extreme biomimetics
In the presence of silicic acid, the team plans to undertake in vitro polymerization of actin monomers into filaments under model circumstances. Hermann Ehrlich and colleagues were able to confirm the existence of actin in silicified skeletal constructions of cold and warm water sponges in this way. The presence of actin as actin-rich axial filaments in Hexactinellida and Demospongiae sponges to form patterns and create the extraordinary structural diversity of biosilica-based skeletons—a source of inspiration in biological materials science—was confirmed by the wide temperature range. Due to the morphological conservation of sponges through fossil records for at least 545 million years, the scientists hypothesized that the involvement of actin was old. They claimed that F-actin evolved in ancestral intracellular structures with the goal of developing advanced 3D biomaterials and novel silicate-containing materials in the lab. The researchers write: “To construct a biomimetic model and explore in-lab mineralization, the idea of generating new silicate-containing materials by employing actin filaments to sustain three-dimensional architectures will be exceedingly exciting.”