Mnemo Therapeutics, a biotechnology organization creating groundbreaking immunotherapies, has declared the distribution of two logical examinations created at Institut Curie, its nearest scholarly partner, in the journal Science Immunology. The distributions that reveal TE-exon joining intersections serve as a source of novel repetitive, disease-explicit targets, with potential implications for developing more viable and less harmful immunotherapies.The discoveries introduced further approve Mnemo’s antigen revelation stage, which is a basic driver of the organization’s cell treatment pipeline.
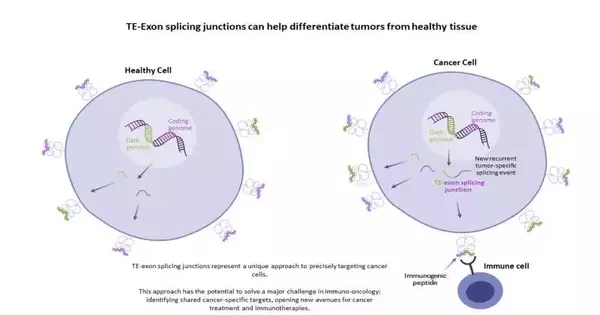
The “dim genome,” otherwise called the piece of the dim genome that is explained, deciphered, and at times interpreted, represents around 45% of the all-out human genome. These genomic locales were generally overlooked because they are underappreciated; however, a new set of proof clues suggests that testing the dim genome could expand the likely universe of previously unknown focuses by recognizing highlights that encode for disease-specific focuses that are both cancer-specific and shared by large numbers of patients.
“Current disease targets start from a tiny level of the human genome, leaving locales with potential oncology targets generally ignored,” said Robert LaCaze, Chief of Mnemo Therapeutics. “The researchers discovered a completely new class of disease antigens that are profoundly growth explicit and repetitive in disease patients by mining the dim genome.””We are anxious to not just better comprehend how these new cancer antigens synergize with our ongoing pipeline, but also the manners in which they may be additionally utilized as a feature of key organizations to propel the more extensive immuno-oncology field.”
“Current cancer targets come from a very limited percentage of the human genome, leaving regions with prospective oncology targets essentially untapped,”
Robert LaCaze, CEO of Mnemo Therapeutics.
In the main review, coordinated by Sebastian Amigorena, Ph.D., senior VP, immunology, and logical prime supporter of Mnemo, CNRS Exploration Chief and head of the Safe Reactions and Disease group (Institut Curie/Inserm), and Marianne Burbage, Ph.D., Inserm Scientist in the group, specialists recognized another group of antigens derived from non-standard joining intersections in mouse cancer cell lines.
These antigens induced a safe reaction in cancer-bearing mice and effectively deferred cancer development when these peptides were managed as prophylactic or helpful immunizations. Moreover, inactivation of Setdb1, a histone methyltransferase, brought about expanded articulation of this group of antigens and cancer cell immunogenicity (the capacity to set off a safe reaction that stops cancer development).
The subsequent review, led by Amigorena and Joshua Cascade, Ph.D., top members of the Integrative Useful Genomics of Disease group (Institut Curie/Inserm), explicitly analyzed this group of antigens in non-small cell cellular breakdown in the lungs (NSCLC) patients and solid tissue tests. The group recognized growth-explicit, non-standard joining intersections that created immunogenic peptides in NSCLC patients, hence depicting a new wellspring of repetitive, cancer-explicit antigens in NSCLC disease patients.
“Recognizing foci that are novel to disease cells and missing from solid tissue has been a significant barrier to developing more fruitful immunotherapies,” Amigorena explained. “The aggregate discoveries advance our insight into growth-explicit antigens, opening additional opportunities for the therapy of disease in the cell treatment space, yet across various methodologies and modalities.”
More information: Marianne Burbage et al, Epigenetically-controlled tumor antigens derived from splice junctions between exons and transposable elements, Science Immunology (2023). DOI: 10.1126/sciimmunol.abm6360. www.science.org/doi/10.1126/sciimmunol.abm6360